Turf Nutrient Management.
This is the second article on Turf nutrient management. We now discuss a factual, unbiased outline of your options. No fancy marketing, glossy brochures or statements without any documentation to support product claims. Instead good old simple facts!
In the first article on high performance turfgrass we discuss:
- Granular vs Liquid Fertilizers for Turf.
- Timing and Frequency of fertilizer use.
- The 4 R’s of Turf nutrient management.
- Soil fertilizer application.
- Foliar fertilizer application.
- How to get the best results.
Topics we now cover on Turf nutrient management include:
- Turf Fertilizer N Forms.
- Light and turf in shade: The impact of these on N Uptake.
- Seasonal Influence: The role of temperature in fertilizer performance.
- Foliar vs soil Uptake of Trace Elements.
- The differences between various inputs and what each can or cannot do.
All of the above are important when it comes to Stadium turf management for out of season events. The right nutrition helps preserve the existing turf and aids its recovery.
In order for turf grass to take up nutrients these must be soluble in water. With a foliar fertilizer, it must also be soluble so it passes through the leaf surface, and into plant cells.
Insoluble mineral salts, which includes all oxides, most hydroxides, carbonates and some sulphates are not taken up by the plant. This means that when you apply these as foliar fertilizers, they just coat the outside of the leaf and do not enter the plant.
After you read this we hope you can make better decisions to produce the best possible turf surfaces.
Nitrogen Forms for Turf.
Below are some of the more common forms of N in use for turf management.
Urea.
This may well be a dirty word to some, but urea is the basis for most of the soluble fertilisers on the market. The reason? It’s cheap and the most cost effective N source available.
Pros: Urea is cheap, and has the highest N content possible (46% N).
Cons:
- It is prone to volatilisation when you use it is a soil application, Data however, does suggest that volatilization losses when you use foliar applications of urea are minimal (1 to 3%).
- During the manufacture of urea several different compounds can form. These include biuret, which is toxic to plants at high concentrations. Urea should contain no more than 0.25 % biuret for safe use as a foliar spray.
- Urea causes soil acidity.
UAN (Urea ammonium nitrate).
Pros:
- UAN has a low potential to volatilise. It is around half as prone to ammonia volatilisation as urea.
- It contains 42% N.
- UAN is tank compatible with other nutrients and pesticides.
Cons: UAN corrodes copper, brass, bronze, galvanizing, and carbon steel.
Ammonium sulphate.
Pros:
- In high pH soils it is beneficial.
- It doesn’t absorb water and “cake”.
- It is soluble.
- Ammonium sulphate is chlorine free.
- It is not prone to leaching, volatilisation or denitrification.
Cons:
- Ammonium sulphate only contains 21% N.
- If you apply too much it can lead to a build up of salt in the soil.
- Too much and it can be toxic.
- There can be issues with tank compatibility.
UF’s and Triazone N.
Microbes breakdown methylene urea and triazone by the methylene urease enzyme. Urea is then converted to ammonium and nitrate.
Pros:
- These have no charge and pass easier through the leaf cuticle than charged molecules like nitrates and ammonium.
- Very low salt index, so they have less chance of burning turf grass.
- UF’s and Triazones have great humectant properties which means they stay wet on the leaf for longer, and increases their time for uptake. In contrast, urea often forms a residue on the leaf surface within 30 minutes of application.
- Triazone N is tank compatible with most post emergent herbicides.
Cons:
- Comparatively expensive.
- Do not make tank mixes with a pH below 5.
Osmolality.
| N Source | Osmolality |
| Triazone N | 533 |
| Urea | 1018 |
| UAN | 1439 |
| Ammonium sulphate | 2314 |
| Potassium nitrate | 3434 |
Ammonium (NH₄⁺) and nitrate (NO₃⁻) N for Turfgrass.
These are the two main forms of N that turf grass uses, and they have different uptake patterns.
How turf grass responds to these N sources depends on factors like the amount of light light (sun vs shade) and the temperature (cool vs warm weather). If you understand these variables it allows you to tailor fertilisation, and promote strong turf growth.
For example, one key difference is that urea and ammonium N enter turf grass leaves more readily than nitrate N.
Ammonium (NH₄⁺):
Pros: Ammonium has a positively charged ion, and binds to soil particles.
- This means that NH₄⁺ is less prone to leach from soils.
- NH₄⁺ is rapidly taken up by turf, and directly converts into amino acids.
Cons: Excessive NH₄⁺ lowers the soil pH over time, and in extreme cases it causes NH₄⁺ toxicity.
Nitrate (NO₃⁻):
Pros: This is a negatively charged ion, and is very mobile in the soil.
- NO3- is easily taken up by turf.
- Turf grass favours NO3- uptake in well-aerated soils.
Cons: NO3- tends to leach, particularly after heavy rainfall or irrigation.
Ammonium vs. Nitrate: Key Differences
Light and Shade: Impact on N Uptake.
Soluble soil NO3- moves via mass flow to the turf roots, and then moves up to the leaves. The energy from the sun then drives the conversion of NO3- N back into NH₄⁺ N which the plant uses to make protein.
Turf Nutrient Management In Sunlight.
Nitrate Absorption.
Full sun favours NO3- uptake. This is because NO3- requires more energy to break down to usable forms within the plant. Photosynthesis provides the energy for this, and so in full sunlight there is more energy available for this process.
Ammonium Absorption.
Although turf grass also absorbs NH₄⁺ in sunlight, it causes excessive leaf growth and also nutrient imbalances. Because it requires less energy to assimilate, it means that under certain circumstances it causes NH₄⁺ toxicity.
Turf Nutrient Management In Shade.
Nitrate Absorption.
Turf grass in shade tends to have lower rates of photosynthesis. This reduces the plant’s ability to efficiently process NO3- N into NH₄⁺ N, and leads to weaker turf growth.
Ammonium Absorption.
NH₄⁺ is the best N source for turf in shade because it does not require as much energy for uptake and assimilation. It is directly converted into amino acids, and provides a more immediate N source for plants in low light levels.
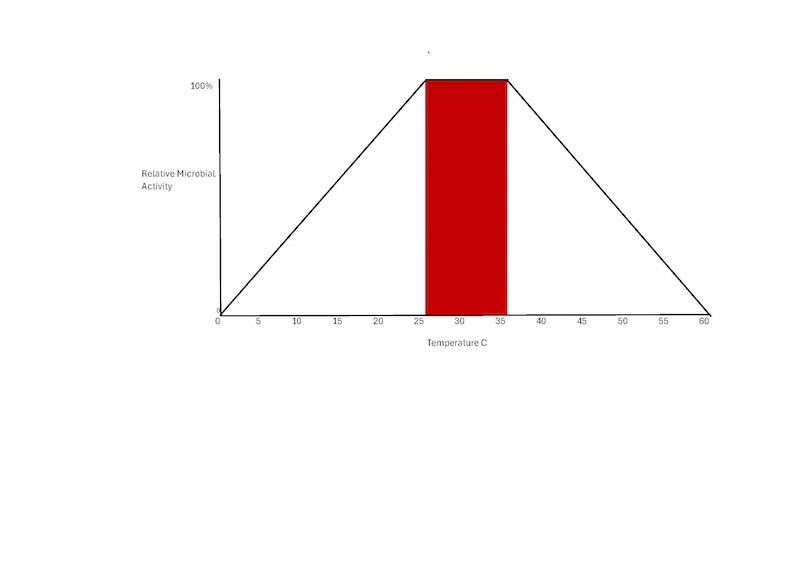
The image below shows how the temperature affects the relative soil microbial activity. There is a peak around 25 to 30 °C and it tapers off above and below this temperature range.
Season: Cool Weather vs. Summer Conditions.
Ammonium vs nitrate in Cool Weather (Spring and Autumn).
Nitrate Uptake.
In cool weather, nitrate uptake is slower because microbial activity in the soil slows down. This in turn reduces the conversion of NH₄⁺ to NO3-.
However, if NO3- is present, cool-season turf grass (like bentgrass and ryegrass) still use it effectively, as they actively grow in cool weather.
Ammonium Uptake.
In cool weather NH₄⁺ is more stable, is readily available for uptake, and is less prone to leach.
You should base turf nutrient management of cool-season turf in the Spring and Autumn around NH₄⁺-based fertilizers. NH₄⁺ provides a steady supply of N to sustain growth whilst temperatures remain low.
Ammonium Toxicity.
- Severe plant chlorosis. This shows as yellow stripes on leaves and looks like an Mg deficiency.
- Lower rates of photosynthesis.
- Turf becomes stunted and has a low shoot to root ratio and
- Less root growth.
How to Counter Ammonium Toxicity.
-
If acid soils are the issue reduce the use of acidifying fertilisers like NH₄⁺ fertilisers, elemental S, and iron sulphate.
-
Use combinations of lime and gypsum. A higher cation presence (especially of Ca), even under low pH conditions, enhances nitrification significantly.
- Incorporate a fraction of NO3- into your fertiliser program. Combinations of urea/NH₄⁺ and NO3-, result in increases in growth of up to 40%.
Summer Fertilisation of warm-season grass (Warm Weather).
Nitrate Uptake.
In warm months, soil microbial activity is at its peak. This enhances the conversion of NH₄⁺ to NO3- (a process called nitrification).
Warm-season grasses (like couch and zoysia) grow best in the Summer, and are able to efficiently use NO3- N.
Ammonium Uptake.
In high temperatures, NH₄⁺ is absorbed but it becomes toxic in excessive quantities. This is likely to occur if uptake exceeds the plant’s ability to use it.
Also, NH₄⁺ has the potential to volatilise. This means it turns into a gas and escapes into the atmosphere if you do not properly water it in.
Practical Recommendations for Turf Managers.
Spring and Autumn Turf Nutrient Management in turf in the sun.
- Use NH₄⁺ fertilisers, for example ammonium sulphate or a balanced mix of N sources. Turf uses both of these options in cool weather, and they have less risk of leaching.
- When turf grass is under stress foliars are less effective and the use of a surfactant is then worth looking at to increase N uptake 1Bethea, F. G., Jr., Park, D., Mount, A., Menchyk, N., & Liu, H. (2014). Effects of Acute Moisture Stress on Creeping Bentgrass Cuticle Morphology and Associated Effects on Foliar Nitrogen Uptake. HortScience horts, 49(12), 1582-1587. Retrieved Dec 30, 2024, from https://doi.org/10.21273/HORTSCI.49.12.1582.
Turf Nutrient Management: Shaded Turf in the Spring and Autumn.
- Prioritize NH₄⁺ fertilisers like ammonium sulphate. This has a lower energy requirement, which means that these work better in shade, when there is less photosynthesis.
Turf Nutrient Management: Sun-Exposed Turf in the Summer.
- Use NO3–based fertilisers like potassium nitrate or mixed N fertilizers like calcium ammonium nitrate. These work better when there is an increase in warm-season grass growth.
Shaded Turf Nutrient Management in the Summer.
- NH₄⁺ remains a good option. Shade means the turf benefits from a readily usable N source, which turf can use without the need for energy hungry photosynthesis.
Foliar vs Soil Trace Element Uptake.
Trace elements, such as Fe, Mn, Zn, and Cu, support plant processes like chlorophyll production, enzyme activation, and overall turf health.
You can supply traces as sulphates, oxides, synthetic chelates, and organic chelates. These all vary in solubility, plant availability, and uptake efficiency by foliar or soil application.
How do these perform when you use them as foliars or soil applications for Turf nutrient management?
Sulphates.
Sulphates are the most common and soluble trace element form. They are low cost and readily available to plants.
Pros:
- Sulphates are very soluble in water. This makes them suitable for foliar and soil applications, as they dissolve easily. The exception to this is calcium sulphate (gypsum).
- They are inexpensive and
- Sulphates are quickly available.
Cons:
- They leach quickly. This is especially the case in sandy soils and
- At high concentrations, when you use these as foliars they can burn turf.
Oxides.
These are less soluble, and have a slower release rate than sulphates. For example, magnesium oxide takes longer to break down and become available than magnesium sulphate.
Pros:
- The oxides are stable in the soil.
- They are less prone to leach. This makes them ideal for long-term soil applications.
Cons:
- Their limited solubility makes oxides inefficient for rapid nutrient uptake. This is especially the case when you use these as foliars where you need quick uptake.
Suspension Concentrates (Oxides/Carbonates)
These have a higher nutrient analysis than chelates, sulphates or nitrates.
- Suspension concentrates are a slow release option. They offer long term nutrient availability.
- They are tank compatible, and cost effective.
- Suspension concentrates are less likely to react in a tank mix.
Chelates.
A chelate does two things:
- Protects a trace element from other chemicals or nutrients.
- Chelates bind to essential metal ions. This increases their solubility and plant uptake.
All chelating agents don’t behave the same.
Synthetic Chelates (e.g. EDTA, DTPA)
Synthetic chelates bind to metal ions. This stops them from dropping out of solution or reacting in the soil. EDTA and DTPA are common synthetic chelates.
Pros:
- Synthetic chelates increase the availability of trace elements. They stop them from becoming immobile or forming insoluble compounds. This enhances foliar and soil uptake.
- They are effective in high pH soils.
- EDTA Chelates are very stable and tank mix compatible. EDTA chelates are stable in the presence of phosphate, and carbonate anions.
Cons:
- Synthetic chelates persist in the soil far longer than you may need, and do not biodegrade.
- They are expensive.
- When you apply these as foliars, synthetic chelates like EDTA, DTPA and EDDHA move slowly into the plant.
- Synthetic chelates have large structures. This is why they have a low analysis.
Organic Chelates (e.g., amino acids, citric acid).
These include amino acids and citric acid, and use natural compounds to chelate traces.
Pros:
- Safer for the environment, and are biodegradable.
- These are gentle on plant tissue. This reduces the risk of leaf burn as foliars.
Cons:
- Organic chelates are less stable than synthetic chelates. This means that they are less effective in extreme pH soils.
- Where claims are made for liquid chelates with an analysis greater than 7.5%, these are not usually fully chelated and may react in tank mixes.
Lignosulphonates.
Lignosulphonates are a lot less stable than EDTA chelates. In actual fact they are only slightly more stable than a sulphate trace element. They have limited longevity in the plant, and require several applications over a season.
Table 1 below provides a quick and simple guide to the benefits of each formulation.
Sulphates | Fulvic acid + Sulphates | Linosuphonates | Suspension Concentrates | EDTA | |
Cost per application | Cheap | Medium | Medium Cost | Getting up there | Expensive |
Turf Safety | Be careful | OK | Be careful | Very | Pretty safe |
Longevity | Don't last | Short term | Don't last | Long Lasting | Pretty good |
Tank Compatibility | Poor | Not the best | Can have issues | Very | Very |
Speed of Uptake | Rapid | Quick | Not bad | OK | Slow |
Soil Uptake of Trace Elements.
For long-term nutrient management soil application is ideal. This builds a reserve of trace elements that plants take up through the roots.
Turf Nutrient Management: Soil Applications:
Oxides: Slow-release oxide forms give a consistent and gradual release of trace elements. They last longer and are less prone to leach. For example, iron oxide supplies iron without the need to reapply.
Synthetic Chelates: Chelates work well in soils with high pH or heavy clay content. This is because they do not become “locked up” in the soil. Synthetic chelates ensure that traces stay in a plant-available form in the root zone, which makes them effective as soil applications.
Sulphates: The sulphates are used in soil applications because of their low cost and high solubility. They give an immediate nutrient release, but are less effective in sandy soils where they are prone to leach.
Less Effective for Soil Applications.
Organic Chelates: The organic chelates are less stable in soil than synthetic chelates, and quickly degrade in extreme pH soils. This means that they are less suitable for long-term nutrient management.
Foliar Uptake of Trace Elements.
If you apply trace elements as foliars, it is an efficient way to quickly correct low nutrient levels, as the nutrients are directly taken in via the leaves.
Turf Nutrient Management: Foliars.
Sulphates.
The high solubility of the sulphates makes them great for foliar sprays. This is especially the case if you want to quickly correct low levels of Fe or Mg. However, you must apply these at low concentrations to prevent leaf burn.
Synthetic Chelates.
Synthetic Chelates (such as EDTA iron) are good as foliars. This is because:
- They are stable.
- Very tank compatible so you can mix more things in your tank at the same time.
- Non-phytotoxic at label rates.
- Readily taken up by turf grass.
- They are really useful to use in high pH soils, that might otherwise limit availability.
Organic Chelates.
The organic chelates are gentle on leaves and effective as foliars. They also enhance improve uptake as they act as surfactants, and give better cover on the leaf surface.
Less Effective for Foliar Applications.
Oxides.
Due to their low solubility oxides, are not effective as foliars. Their low solubility means they are not efficient at entering the leaf. Oxides are best to apply to the soil.
Recommendations for Turf Nutrient Management.
Turf Managers are well aware that two trace elements that provide excellent colour are Fe and Mn. However, to get a better colour response:
- In general, cool-season turf prefers a 3:1 ratio of Fe to Mn for a better green colour.
- If you add a little ammonium N with Mn it gives a slightly faster response.
Foliar Applications.
- Use synthetic or organic chelates for efficient, safe foliar uptake. Synthetic chelates are excellent for a rapid correction in alkaline soils, while organic chelates are better in environmentally sensitive areas.
- Low concentrations of sulphates quickly address deficiencies. For example you can address Mg deficiencies with fast-acting nutrients like magnesium sulphate (Epsom salts).
- Be cautious of leaf burn with you use high concentrations of sulphates.
- Aim to apply foliars early in the morning in humid conditions to get the best results. This is because the droplet remains wetter for longer which gives a longer time for uptake.
- For the best results Turf Managers should allow foliars to sit on the leaf for few hours before you syringe or irrigate. If you need to water the turf, nutrients can still be taken up by the roots. You are simply washing any fertilizer left on the leaf into the rootzone.
Soil Applications.
- Choose oxide forms for slow-release soil applications. This is important where you need a consistent source of nutrient over time.
- Use synthetic chelates in high pH soils where other forms of trace elements might become unavailable. Chelated iron, for example, works well in alkaline soils to prevent Fe chlorosis.
- Apply sulphates when you want an instant response. This might be the case in sandy soils with a high potential to leach.
- If you want to quickly correct low trace element levels then sulphates are the way to go. However, you need to often reapply these in sandy soils.
Key Takeaways for Turf Managers.
If you understand the differences between foliar and soil uptake, and the properties of trace element fertilisers, you are better able to develop the effective fertilisation strategies for turfgrass.
- Spray volume effects the foliar uptake of urea. The higher the spray volume the less the uptake of urea N. This raises how effective “hose on applicators” sold into the home garden market are to apply “foliar” nutrition. The high water volume in use means that this is fertigation and not truly foliar.
- No matter what the spray volume less than 20% of the urea is taken up via the leaf. Thats why it’s important to syringe after a foliar application to wash fertiliser off the leaf and into the rootzone. Once you wash it into the soil, ammonium N undergoes microbial action to convert it to nitrate.
- Spray adjuvants, improve foliar uptake.
References

Jerry Spencer
Jerry has an Hons Degree in Soil Science (1988) from Newcastle Upon Tyne University. He then worked as a turf agronomist for the Sports Turf Research Institute (STRI) until 1993.
He gained a Grad Dip in Business Management from UTS in 1999. He has held a number of technical roles for companies such as Arthur Yates (Commercial Technical Manager) and Paton Fertilizers (Organic, turf specialty and controlled release fertiliser) portfolios.
In 2013 he established Gilba Solutions as independent sports turf consultants and turf agronomists. Jerry has written over 100 articles and two books on a wide range of topics such as Turf Pesticides and turfgrass Nutrition which have been published in Australia and overseas.