Urease Inhibitors.
This is the second blog on slow-release fertilizers: nitrogen stabilizers in turf. The first is on nitrification inhibitors and this covers the stabilized nitrogen (N) urease inhibitors. Both of these fertiliser technologies are in common use in Australia.
Stabilized nitrogen Urease inhibitors are in a turf managers ‘tool box’ of products that save you time and money. This ‘tool box’ includes Sirflor 38.
Products like these help you to produce high quality surfaces at a minimal outlay. There are now in fact, several options available for turf grass.
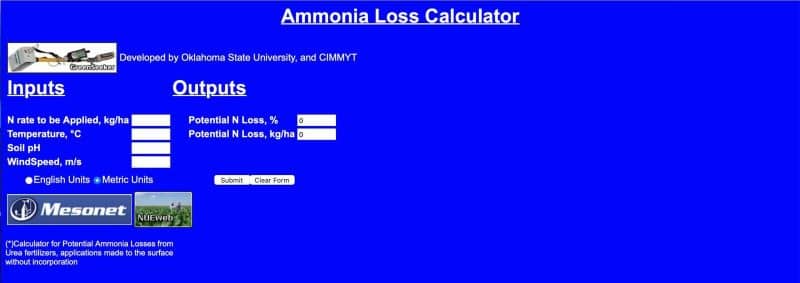
At the end of this article is a free ammonia loss calculator, which can demonstrate potential losses from your site.
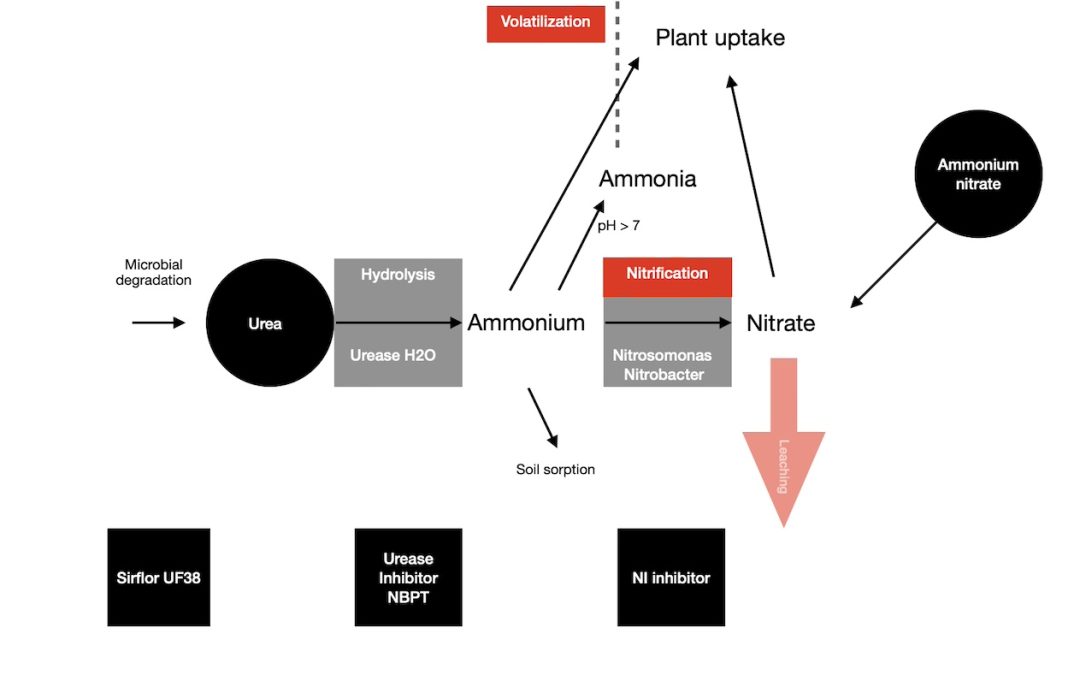
How do Urease Inhibitors stop Volatilization?
When you apply urea or ammonium fertilizers to a soil, they can lose N due to ammonia volatilization. This is often the case with urea and liquids like urea ammonium nitrate (UAN).
Stabilized nitrogen Urease inhibitors stop or slow down the transition of urea to ammonium hydroxide and then to ammonium. Because they slow down the rate of urea hydrolysis in the soil, this reduces the loss of ammonia.
Factors That Affect Urea Volatilization.
- Evaporation Rate.
- Soil Moisture and Rainfall.
- Critical Relative Humidity (CRH).
- Soil pH.
- Temperature.
- Soil texture.
- Thatch levels.
Urease Inhibitors and Evaporation Rate.
Factors that increase evaporation will tend to increase volatilization losses. As ammonia moves to the soil surface due to evaporation, it causes an increase in the concentration of ammonia in the soil solution. Windy conditions further increase this loss of moisture and in turn volatilization losses.
Soil Moisture and Rainfall
When you apply urea to a soil surface, volatilization increases as the soil water content increases. This volatilization continues until the soil becomes saturated.
This contrasts with when you apply a granular fertiliser to dry soil. In this case volatilization doesn’t occur, because the fertiliser granules have to dissolve before any volatilization losses occur.
Once the fertiliser granules dissolve, volatilization increases as water evaporates from the soil. This moisture loss due to evaporation then increases the concentration of ammonia at the soil surface.
Volatilization losses are high If you apply urea to a moist soil, and the soil then dries out.
If significant rainfall or irrigation occurs after you apply a fertiliser it will reduce volatilization. The water dilutes the ammonia N, and so lowers the ammonia concentration at the soil surface.
To prevent volatilization when you use urea you need to apply at least 6mm of water to leach urea deep enough into the soil,
Critical Relative Humidity (CRH).
When the humidity in the turf canopy is greater than in the atmosphere, urea will dissolve.
As you may be aware the solubility of urea is high. In fact at 20°C, Over 1 Kg (1079 g) of urea will dissolve in a litre of water.
When you spread granular urea it very hygroscopic. Urea absorbs moisture from the air, and also from a wet soil. In a turf canopy it is also subjected to dew, humidity, rainfall and irrigation.
In order to dissolve in these circumstances, the CRH must be exceeded.
The CRH is the relative humidity of the atmosphere (at a specific temperature). It is when a fertiliser starts to absorb moisture from the air. Below this temperature it doesn’t absorb moisture from the air.
- The CRH of most fertilisers decreases as the temperature increases.
-
Mixtures of salts usually have a lower CRH than the individual fertilisers themselves.
-
Fertilisers that contain urea have a much lower CRH than fertilisers that do not contain Urea.
-
At a temperature of 30°C, the CRH for urea is 72.5%.
Urease Inhibitors and Soil pH.
High soil pH increases volatilization.
- At a high soil pH there is an increase in soil water ammonia concentrations.
- Soil water is able to hold less ammonia gas the warmer the soil water gets.
When you use a granular urea fertilizer it increases the soil pH in a 25 mm radius of the granule. This then causes N volatilization to increase which leads to an increase in losses.
This pH increase is much higher in high sand content root zones because of their lack of any buffering capacity, in comparison to heavier soils.
The pH rise around a granule of urea increases the conversion rate of ammonium to dissolved ammonia. This in turn increases the amount of ammonia that can volatilize.
Although this is a temporary pH increase, it results in volatilization losses from soils with a pH as low as 5.5.
An increase in a soil pH from 6.5 to 7.5, doubles volatilization from 10 to 20% if you leave urea on the surface for four days.
Urea fertilizers plus a stablized nitrogen urease inhibitor, slow this conversion of urea to ammonium, and also minimise any pH increase.
Temperature
Warm temperatures increase volatilisation. When the temperature increases it does two things.
- It increases the rate of urea hydrolysis.
- It increases the converstion rate of ammonium to ammonia.
An increase in temperature from 7°C to 16°C doubles the loss from volatilization at the same soil moisture content.
Soil Texture.
Ammonia losses tend to be higher from coarser than from finer-textured soils. This is because fine-textured soils tend to have a high CEC and so are able to hold onto more ammonium.
Soil factors that reduce volatilization include:
- High clay content.
- High CEC soils.
- Soil organic matter.
- Bicarbonate content.
Urea volatilization is almost 3x higher in sandy loam soil with a CEC of 7, than in a silt loam soil with a CEC of 12.1.1Keller GD. and Mengel DB. 1986. Ammonia volatilization from nitrogen fertilizers surface applied to no-till corn. Soil Science Society of America Journal. 50:1060-1063.
7. Urease Inhibitors and Thatch.
High levels of thatch increase volatilization.
- In order for urea hydrolysis to occur, soil microbes need to produce the urea enzyme. Urea enzymes are 40 times more active in surface organic material than in a mineral soil2Torello W and Wehner DJ. 1983. Urease Activity in a Kentucky Bluegrass Turf. Agronomy Journal 75(4) DOI:10.2134/agronj1983.00021962007500040018x .
- Thatch tends to hold water. This increases ammonia in the solution that can volatilize.
- Surface thatch stops N from moving into the soil.
Urease Inhibitors on the Market
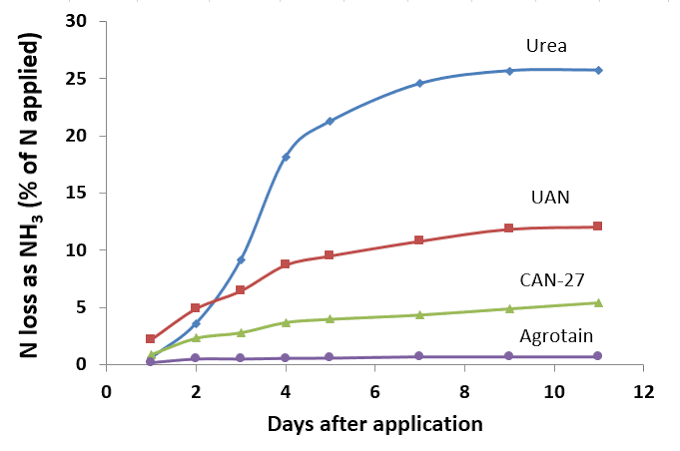
Common stabilised nitrogen urease inhibitors in Australia, include NBPT, which is the active in Agrotain. This nitrogen stabilizer inhibits the urease enzyme but does not always result in an increase in turf growth.
Increases in turf growth only occur if you reduce volatilization losses from surface-applied urea when:
- The amount of N limits growth.
- Losses from volatilization are so high that they have an impact on turf growth.
You always get the most benefit from these products, when the existing N levels are low, and conditions will cause volatilization losses.
You don’t need to use a urease inhibitor:
- If you apply at least 6mm of water straight after you apply a fertiliser. In this case volatilization losses are very low.
- If it’s going to rain straight after you fertilise.
Use of these products significantly reduces N losses due to volatilization4Reducing N Fertilizer Loss to Air, Montana State University, https://landresources.montana.edu/soilfertility/soilscoop/ss_UVol.html.
Dual Inhibited Products
This nitrogen stabilizer technology combines urea plus a nitrification and a urease inhibitor. There are two commercially available options on the market.
Alzon® Neo-N Fertilizer.
Alzon® Neo N is a dual-inhibited N turf fertilizer that contains 46% N. It lasts for up to 10 weeks.
- It is very soluble. You can use this as a dry granule or dissolve it in a spray tank. As a soluble rates are as low as 25 Kg/Ha (2.5g/m2).
-
Alzon Neo N isn’t a coated fertilizer. The inhibitor is present through the entire granule. This means the inhibitors do not ‘rub off’ in transit or if you are blending.
-
This increase in N efficiency, means you can use low rates with no loss in turf quality.
-
When you use Alzon as a soluble it doesn’t leave any tank residues.
-
Research shows that the nitrification inhibitor in Alzon Neo-N reduces leaching by up to 50%. This saves you money, whilst also results in great looking turf.
Umaxx and Uflexx.
- Umaxx and Uflexx combine a urease inhibitor plus an NI, with urea. These stabilizers are NBPT, and “Didin”.
- NBPT stops N losses from volatilization. The result of this is that it keeps the N stable for longer.
- Umaxx is the premium turf-grade N stabilizer. It is a granular stabilized urea and comes in two particle sizes: SGN 150 and 237. It claims to have a longevity of 12 to 16 weeks.
- The other turf grade product is Uflexx. This comes in the same sizes as Umaxx, but contains less of the nitrogen stabilizer. It claims to last 6 to 8 weeks.
- Umaxx and Uflexx contain the same amount of NBPT, and it is the NBPT that stops volatilisation for up to 14 days.
- You can use these in dry fertilizer blends, as stand-alone applications or as a solubles.
When to use Nitrogen Stabilizers.
Stabilized Nitrogen products are not for everyone. However they can save a lot of money and improve the results you get when you fertilise.
As with most things be aware that in many cases you can pay for something that you don’t need and N stablises are no excpetion to this.
Nitrogen Stabilizers are not for all situations. However, the flip side of this is that in some cases, a nitrification or a urease inhibitors is a good option to save money and give yourself a good chance to present a better quality surface.
Conclusion.
-
Use a slow release fertilizers like UF38 in the summer and IBDU in the Autumn. This will reduce N losses due to volatilization.
-
Thatch does not just effect moisture movement into the soil profile. Excessive thatch costs you money as any N fertilizer you apply isn’t used efficiently.
When to use an N stabilizer | |
Urease Inhibitor (NBPT) | Nitrification Inhibitors (DMPP, DCD) |
Neutral and alkaline soils >pH 7 | Highly saturated/waterlogged soils |
Excessive thatch | Heavy, poorly aerated and compact soils |
Soils with low cation exchange capacity (CEC)/ Poorly buffered soils (low organic matter, content, high sand content) | Sandy soils that are prone to leaching |
Heavy, compacted and poorly aerated soils | |
Applications where conversion of Ammonium to Nitrite can still take place. (temperatures of +4°C or warmer) | |
References

Jerry Spencer
Jerry has an Hons Degree in Soil Science (1988) from Newcastle Upon Tyne University. He then worked as a turf agronomist for the Sports Turf Research Institute (STRI) until 1993.
He gained a Grad Dip in Business Management from UTS in 1999. He has held a number of technical roles for companies such as Arthur Yates (Commercial Technical Manager) and Paton Fertilizers (Organic, turf specialty and controlled release fertiliser) portfolios.
In 2013 he established Gilba Solutions as independent sports turf consultants and turf agronomists. Jerry has written over 100 articles and two books on a wide range of topics such as Turf Pesticides and turfgrass Nutrition which have been published in Australia and overseas.