The Role of Sulphur in Plants and Turfgrass.
Sulphur (S) in plants is essential for growth and healthy soils. We discuss S fertilisers such as thiosulphate and the role of the sulphate ion in S uptake. Turf needs this element in smaller amounts than the primary elements like ,N P, and K.
Sulphur is vital for:
- The synthesis of important amino acids.
- It directly supports protein construction and metabolic functions.
- It improves turf health. Turf deficient in S is chlorotic (yellow), stunted, and is less tolerant to wear.
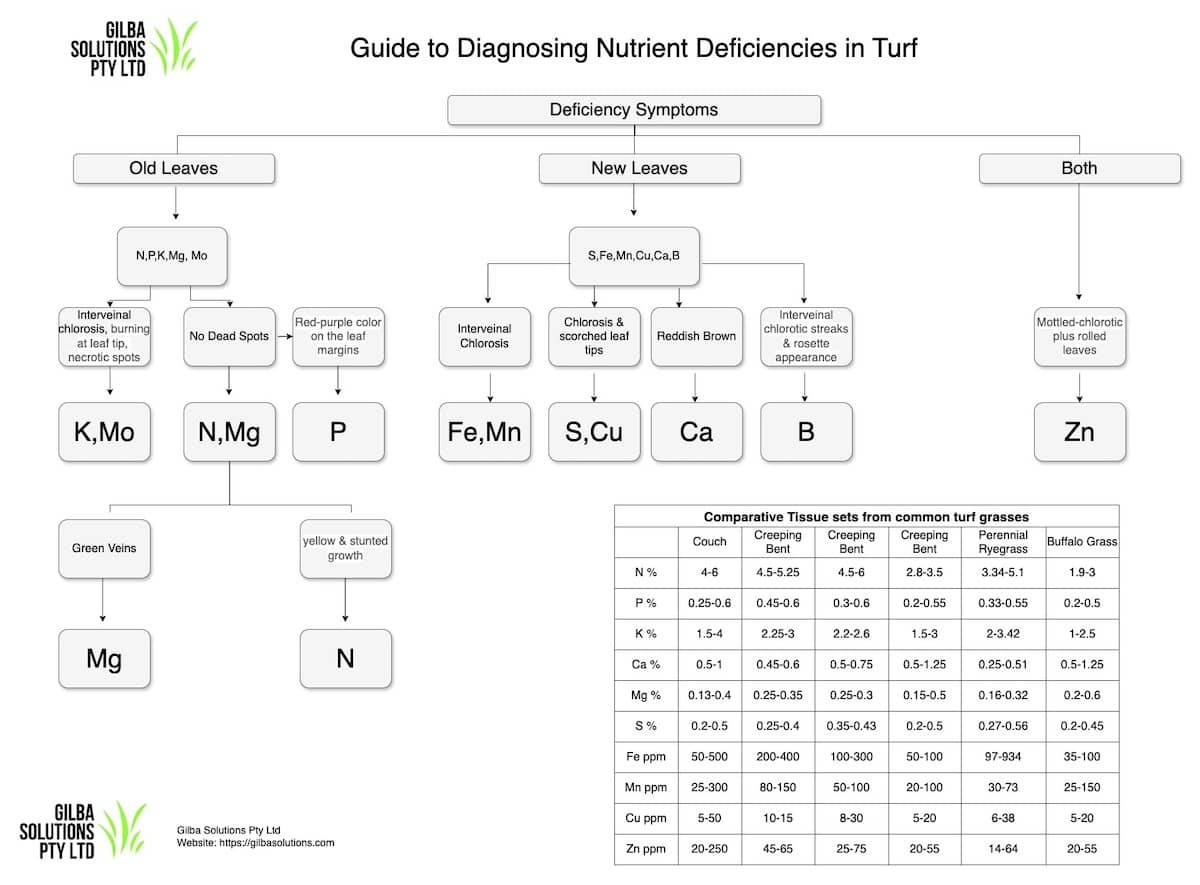
- Deficiency symptoms of Sulphur in plants first appear as leaves with yellow leaf tips and brown edges.
- Sulphur in the soil helps with the soil structure. It stimulates microbial activity, supports nutrient uptake, and helps regulate soil acidity.
Sulphur in Soil.
- Plants mainly take this up in the form of the sulphate (SO₄²⁻) ion.
- It is mobile in the soil. This is especially the case in sands or alkaline conditions.
- It can leach. This means that you need to regularly test for this. Especially on sand based constructions like sports turf, golf courses, and sandy soils.
- S helps to reduce the soil pH (increase acidity). This can improve the availability of some trace elements in alkaline soils.
- More than 95% of the soil S is tied up in organic matter.
- In the soil it forms sulphuric acid due to microbial oxidation. Over time this acidifies the root zone.
The Sulphate Ion.
- Generally the sulphate ion does not strongly absorb to soil colloids or organic matter. The exceptions to this are when the soil pH is low or Fe and Al oxides are present.
- It tends to stay in the soil solution and moves with soil water. This means it readily leaches in sandy soils, where there is high rainfall or irrigation.
- In contrast it builds up in heavy soils that are not irrigated or there is low rainfall. This remans available to deep rooted turf grass.
Causes of Sulphur Deficiency.
Mowing.
When you mow and box off the clippings you physically remove Sulphur in plants.
Organic Matter.
- Soils that have less than 2% S are likely to be deficient. However, you can see deficiencies in soils with much higher S levels. As a rough guide for each 1% of OM, mineralisation releases about 6 Kg of Sulphur/Ha per year1Schoenau, JJ. and Malhi, SS. (2008). Sulfur Forms and Cycling Processes in Soil and Their Relationship to Sulfur Fertility. In Sulfur: A Missing Link between Soils, Crops, and Nutrition, J. Jez (Ed.). https://doi.org/10.2134/agronmonogr50.c1.
Soil Texture.
- Sulphate is more likely to leach in high sand content soils than heavy soils.
- You tend to find that turf gives more of a response to Sulphur fertilisers in coarse, well drained sand root zones.
- In the Spring you often see it is associated with a turf requirement for K.
Irrigation Water.
- Irrigation water can vary in the amounts of the sulphate ion that it carries.
- This means that a regular irrigation suitability analysis is recommended.
Symptoms of Sulphur Deficiency in Plants.
- A deficiency of Sulphur in plants is first seen in young leaves. These will show signs of chlorosis (yellowing of the leaves). The parallel leaf veins remain green and the surrounding tissue becomes yellow. This is in contrast to N deficiency which you tend to see in the older leaves first, and where the veins are yellow.
- Growth is stunted.
- Turf tends to have fewer new shoots or tillers.
- In some cases you can see the leaf sheaths become purple or red.
Sulphur and Soil Acidification.
In a highly buffered soil (high clay) it can be difficult to change the pH. In contrast you can change the pH of a soil with a low buffering capacity (a sand) much more easily.
The reason for this are those soils that contain lime (calcium carbonate) which are called calcareous soil.
Many soils, contain high levels of calcium carbonate (CaCO3 ). In fact many sand-based constructions are often made of sands that contain high levels of calcium carbonate.
When you try and lower the soil pH in a highly buffered soil, you tend to see a temporary drop in the pH. However, this quickly returns to the buffer pH when you stop the use of Sulphur.
From a turf management perspective, when the soil pH is high in a buffered soil some traces will be unavailable to the turf. In this situation rather than try to lower the soil pH, it’s better to apply liquid trace elements to the foliage.
Our blog discusses the use of Sulphur in the soil and acidification to manage Winter Grass.
Black Layer.
You often find black layer on turf areas, and especially sand based constructions. It is a dark to black layer that contains sulphides and is caused by anaerobic bacteria.
When the soil is waterlogged and low in oxygen, the bacteria produce hydrogen sulphide gas. This gas is a result of their metabolism.
The hydrogen sulphide can react with metals in the soil, and form metal sulphides. You see this as a black, smelly layer in the soil that smells of “rotten eggs”.
Black layer can negatively affect turf health. Hydrogen sulphide gas is toxic to plant roots, and the layer limits water and nutrient uptake. You see the symptoms as yellow, thin areas and a general decline in turf quality.
Years ago when I was working in VIC, the approach to manage this was to stop the use of S. This undoubtedly lead to low Sulphur levels in the turf.
Instead, as it’s a problem of anaerobic soils, the key to manage this is aeration. The cause of black layer is not the soil S. In order for black layer to form the bacteria need anaerobic soils.
If you adopt a program of regular aeration, it helps oxygen get into the soil, and water move away from the surface. The Increase in soil oxygen inhibits the bacteria, and reduces the production of hydrogen sulphide.
Sulphur Fertilisers.
There are several Sulphur fertilisers that you can use in turfgrass management. Each has a different analysis, acidifying effect, and solubility.
- Ammonium Sulphate. This provides N and S. It acts quickly, and is highly soluble. It only has a moderate affect on the pH.
- Elemental S. This is very effective to lower the soil pH over time. It achieves this by microbial oxidation. However, it is insoluble in water, slow, and needs the activity of soil microbes to work.
- Gypsum (Calcium Sulphate). Gypsum supplies Ca and S without effecting the pH. It is moderately water-soluble.
- Magnesium Sulphate. It delivers Mg and S, is very soluble, and has a minor pH effect.
- Potassium Sulphate. Supplies K and S. It has a neutral or slight acidifying effect. It is soluble in water.
- Potassium thiosulphate (KTS). Potassium thiosulphate provides K and S as thiosulphate. It is highly soluble and immediately available for uptake.
Sulphur in the soil converts to sulphate in 1 to 3 weeks2Sharma RK, Cox MS, Oglesby C, Dhillon JS. 2024. Revisiting the role of sulfur in crop production: A narrative review. Journal of Agriculture and Food Research Volume 15, March 2024, 101013 depending on the soil conditions. Thiosulphate acidifies the soil, and also enhances trace element availability (Fe, Zn, Mn, and P) as it increases their solubility.
It also acts as a nitrification inhibitor. KTS slows down the conversion of ammonium to nitrate, and reduces N leaching. This increases N use efficiency.
If you over use S fertilisers it can lead to excessive soil acidification. This can result in an increase in the solubility of potentially phytotoxic elements like Al.
Here is a table of the common S fertilisers in Australia. This includes Potassium Thiosulphate (KTS). It outlines their analysis, solubility, and effects on soil pH:
Table of Fertilisers to Address Deficiencies of Sulphur in Plants and the Soil.
| Fertilizer | Nutrient Analysis (Approx.) | Solubility | Soil & pH Effect |
|---|---|---|---|
| Potassium Thiosulphate (KTS) | 30% K w/v and 25% S w/v | Highly soluble in water | Thiosulphate rapidly oxidises to sulphate. It initially has a near neutral pH but when it oxidises it acts as a soil acidifier. It helps lower high soil pH, improves trace element availability (Fe, Zn, Mn), and acts as a nitrification inhibitor. It delays the conversion of ammonium to nitrate N. |
| Ammonium Thiosulphate (ATS) | ~16% N and 34% S w/v. | Highly soluble | This is similar to KTS. It quickly converts to sulphate, and acidifies the soil. It slows down urea hydrolysis and nitrification, and reduces N losses. |
| Ammonium Sulphate | 21% N and 24% S. | Soluble | Immediately plant-available sulphate; mild acidification; fast sulphur supply. |
| Iron Sulphate | 19.5-20% Fe and 11.5% S | Moderate solubility | Immediately plant-available sulphate; mild acidification; fast sulphur supply. |
| Magnesium sulphate (Epsom Salts) | 9.6% Mg and 14% S. | Soluble | Immediately plant-available sulphate; mild acidification; fast sulphur supply. |
| Potassium Sulphate | 41% K and 18% S. | Soluble | Immediately plant-available sulphate; mild acidification; fast sulphur supply. |
| Gypsum | 23% Ca and 12-18% S | Moderate | Provides an initial increase in available S. Then it slowly releases S over time. |
| Elemental Sulphur (S) | 100% S (elemental) | Insoluble. It needs microbial oxidation to occur. | Elemental S slowly oxidises to sulphate S over weeks and months. It acidifies the soil by producing sulphuric acid. It is used to lower the soil pH in high pH soils but acts slowly. |
Summary Notes:
-
The liquids ATS and KTS, are very soluble in water and colourless. The form of K and S that these supply quickly oxidises to sulphate S. It acidifies soil once it oxidises, and helps lower the pH of alkaline soils. When it does this it increases the availability of some trace elements, inhibits nitrification and stabilises N.
-
S fertilisers in the sulphate form are immediately available to turf plants. This is unlike elemental S, which requires microbial conversion to work.
-
As KTS and ATS inhibit nitrification they can slow N losses. This improves fertiliser efficiency.
Elemental Sulphur.
- Although elemental S is poorly soluble, it is an important source of S. Before turf can use this must oxidise the S to the sulphate form.
- This conversion occurs mainly as a result of the activity of bacteria called thiobacilli3Das S and Dash HR. 2020. Microbial and Natural Macromolecules Synthesis and Applications. ISBN 978-0-12-820084-1.
Conditions that Favour Oxidation:
- The soil temperature.
- Soil moisture.
- Low soil pH.
- Aeration.
- Particle Size.
Particle size plays the most important role. Agronomically, large particles that are greater than 0.5 mm in size are of little value. To get the best results you need to use finer particles that are less than 0.25 mm in diameter.
Soluble Sulphate Fertilisers.
The water soluble sulphate fertilisers such as ammonium sulphate and potassium sulphate provide an immediately available solve of sulphate ions.
These are best to use to correct an existing soil deficiency or when you need a quick response.
References

Jerry Spencer
Jerry has an Hons Degree in Soil Science (1988) from Newcastle Upon Tyne University. He then worked as a turf agronomist for the Sports Turf Research Institute (STRI) until 1993.
He gained a Grad Dip in Business Management from UTS in 1999. He has held a number of technical roles for companies such as Arthur Yates (Commercial Technical Manager) and Paton Fertilizers (Organic, turf specialty and controlled release fertiliser) portfolios.
In 2013 he established Gilba Solutions as independent sports turf consultants and turf agronomists. Jerry has written over 100 articles and two books on a wide range of topics such as Turf Pesticides and turfgrass Nutrition which have been published in Australia and overseas.

