Not all granular gypsum is the same
As we head towards Spring thoughts for some will be turning towards applying gypsum. In the turf industry several companies supply granular gypsum products. Spreading gypsum is a lot easier when it is a granule, and this is the reason why these granular forms of gypsum have become more popular.
Spreading gypsum adds calcium ions (Ca 2+) to the soil and this also acts as a clay breaker. In order to work properly these must be soluble.
Granular gypsum should be in the calcium sulphate dihydrate form. There is a really good reason for this, and why it should only contain this and no “bells and whistles”
Gypsum vs gyprock.
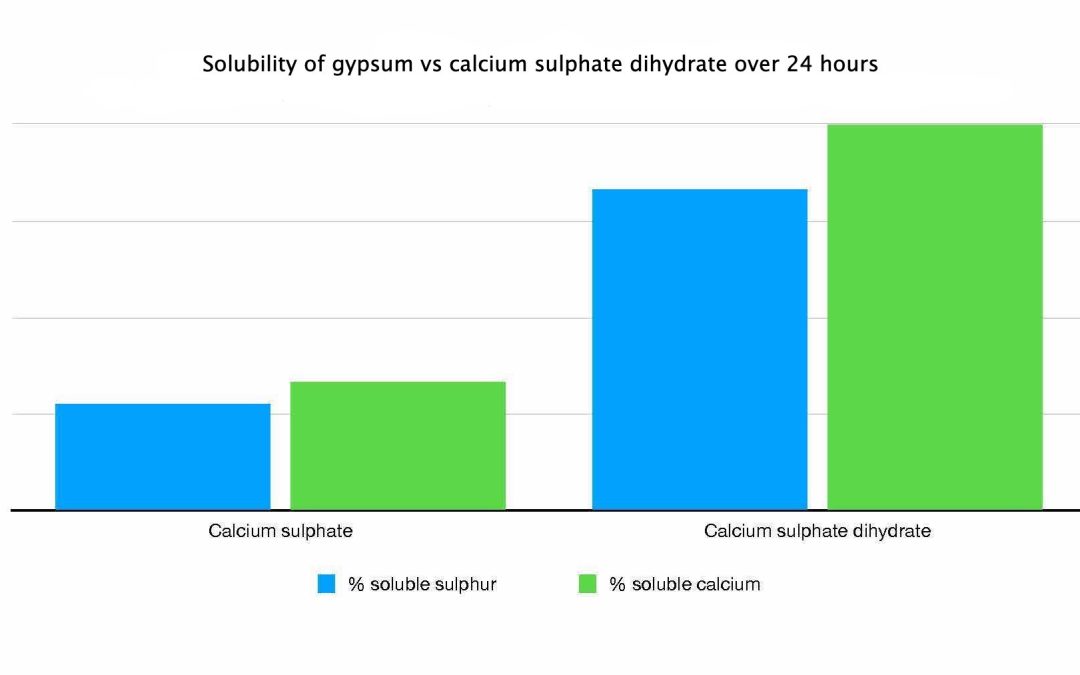
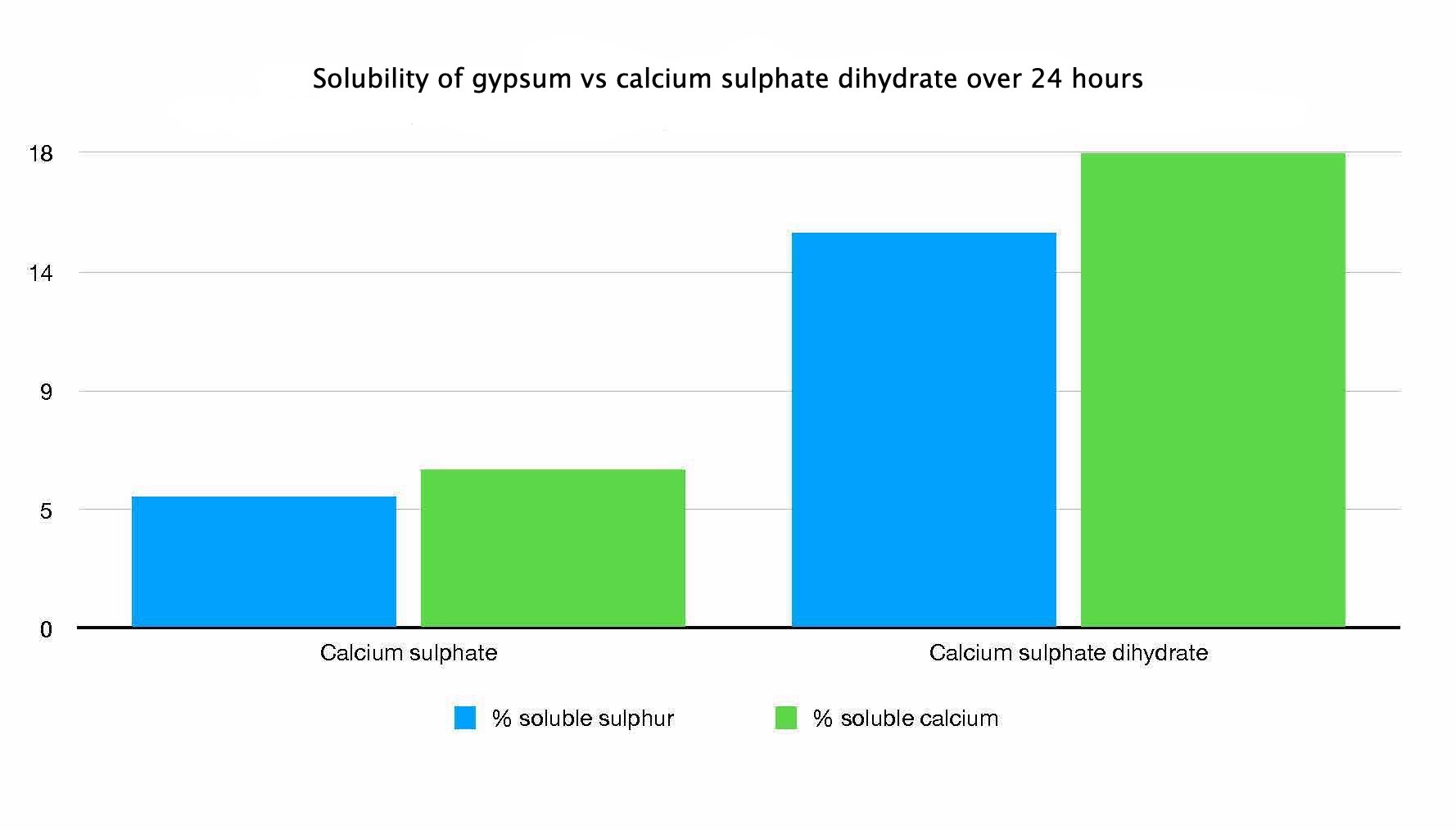
Granular gypsum has the chemical formula of CaSO4 (2H2O). This means that each gypsum molecule contains one Ca2+ cation, one SO4 2- anion, and two waters. There are however, other calcium sulphate based products that are sold as clay breakers. These are based on calcium sulphate anhydrite (CaSO4), which is a key component of gyprock.
Chemically, these two salts are similar, but the main difference is that calcium sulphate anhydrite doesn’t contain water. Consequently, calcium sulphate anhydrite contains more calcium on a weight basis than gypsum.
Calcium sulphate anhydrite contains 29.4% Ca, whereas gypsum contains 23.2% Ca. However, we are interested two things.
- Firstly, the solubility of the granular gypsum product (how much of the salt will dissolve in the soil water) and
- Secondly, the rate of dissolution (how fast the salt dissolves in water).
The addition of the 2 waters make the dihydrate form more water soluble. This means it works a lot faster in comparison to the anhydrite form. So in reality you are best to use the anhydrite form for gyprock and cement.
The rate of dissolution is important for dealing with soil crusting, due to the dispersion of clay particles at the soil surface. In this case, rapid dissolution is important to maintain a high level of dissolved Ca2+ in the surface soil. This is because rain or irrigation water tends to leach cations from the uppermost layer of soil.
Published reports indicate that the dissolution rate of calcium sulphate anhydrite is 5-72% slower than that of the dihydrate form.
However, for general treatment of soil structure, the rate of dissolution is less important than the overall solubility.
Interestingly in many states in the USA fertilizer labelling regulations restrict the use of the word “gypsum” to granular gypsum products composed primarily of CaSO4*2H2O (calcium sulphate dihydrate).

Jerry Spencer
Jerry has an Hons Degree in Soil Science (1988) from Newcastle Upon Tyne University. He then worked as a turf agronomist for the Sports Turf Research Institute (STRI) until 1993.
He gained a Grad Dip in Business Management from UTS in 1999. He has held a number of technical roles for companies such as Arthur Yates (Commercial Technical Manager) and Paton Fertilizers (Organic, turf specialty and controlled release fertiliser) portfolios.
In 2013 he established Gilba Solutions as independent sports turf consultants and turf agronomists. Jerry has written over 100 articles and two books on a wide range of topics such as Turf Pesticides and turfgrass Nutrition which have been published in Australia and overseas.